The Peregrine System™ Infusion Catheter (Peregrine Catheter)
The Peregrine Catheter uses a proprietary patented technology to deliver diagnostic and therapeutic agents directly to the perivascular space that surrounds blood vessels. The catheter is intended for the infusion of diagnostic and therapeutic agents into the perivascular area of the peripheral vasculature.
Targeted Perivascular Delivery of Solutions
The Peregrine System Infusion Catheter features three guided microneedles for targeted infusion. The catheter is delivered into the femoral artery utilizing techniques similar to coronary or peripheral catheterization. During endovascular catheter placement, the microneedles remain retracted within radiopaque guide tubes, enabling fluoroscopic confirmation of catheter positioning and apposition of the guide tubes prior to delivery of the diagnostic or therapeutic solution. Once positioning and apposition has been confirmed, all three micro-needles are deployed simultaneously for controlled delivery of the diagnostic or therapeutic agents directly into the perivascular space.
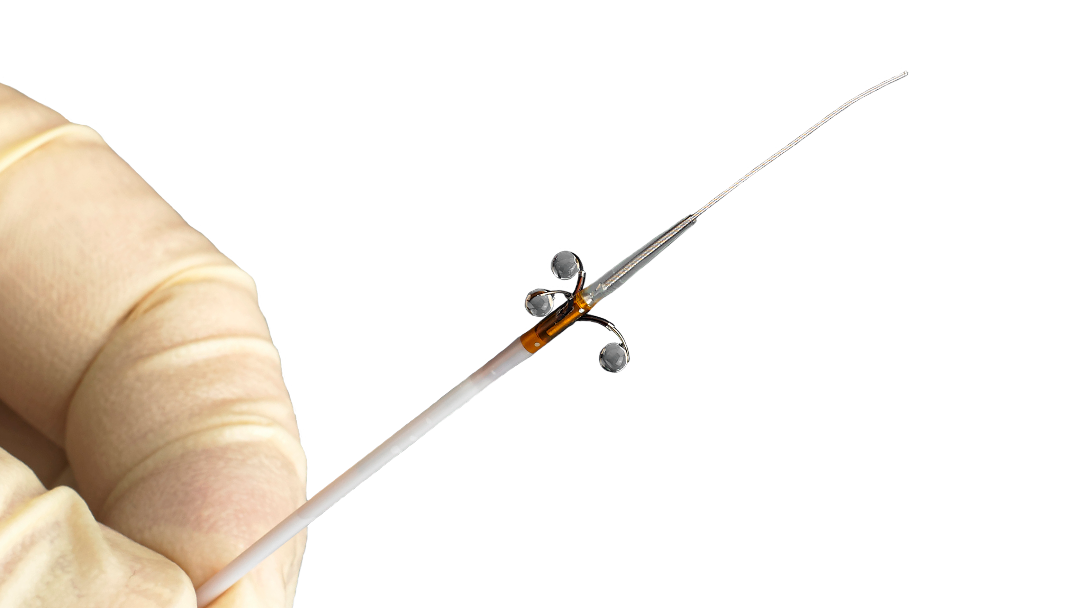
The Peregrine System Infusion Catheter is intended for the infusion of diagnostic and therapeutic agents into the perivascular area of the peripheral vasculature.
